The Members React With Metals to Form Salts
Correct option is A Displacement reactions are very common in metals. Reaction Between Metals and Acids.

Periodic Table One Of The Most Important Tools Of The Chemist Is The Periodic Table Of The Elements It Is Used For Element Identification Element Masses Ppt Download
Lead and the metals ranking above lead on the activity series form salts when reacted with hydrochloric acid or sulphuric acid.

. 1 CuSO4 aq Zn s - Cu s ZnSO4. The halogens react with metals to produce salts the word halogen means salt former. Not entirely of course but this fairly electronegative metal does form a compound with cesium whose structure resembles an alkali metal halide.
The reaction between zinc and. This class experiment is often used in the introductory study of acids to establish that this behaviour is a characteristic property. Sodium chlorine sodium chloride.
Lithium is the only metal that reacts directly with carbon to give dilithium acetylide. The term halogen means salt former. List the metal salts that form acidic solutions 4.
Dative covalent bond and the metal ion is the Lewis acid. With hydrogen alkali metals form saline hydrides that hydrolyse in water. Group 7 elements form salts when they react with metals.
The experiment is done first on a smaller scale using test tubes lesson 1 below with no attempt to recover the salts formed. Metals react with oxygen in the air to form oxides. It is more reactive than the other halogens.
2Na s Cl2g. These reactions also involve the liberation of hydrogen gas. Such reaction is known as displacement reaction or single displacement reaction.
Reaction of metals with salt solutions Metal A. Na and K can react with acetylene to give acetylides. It reacts with the alkali metals M to form a salt MX where X is the halogen.
Write the chemical reaction equations of each metal ion combining with water to make the solution acidic. There are other nonmetals that can be mixed with metals. It is simple and easy if metal A displaces metal B from its solution it is more reactive than B.
For example chlorine reacts with sodium. It forms interhalogen compounds only of the form XA where A is the unknown and X is another halogen. Reaction of metal with salt solution.
Write the chemical reaction equations of each metal ion combining with water to make the solution acidic. This establishes that hydrogen production is a. So as soon as hydrogen gas is formed in the reaction between a metal and dilute nitric acid the nitric acid oxidizes this hydrogen to water.
Its hydrogen halide HX forms a weakly acidic solution. List the metal salts that form acidic solutions 4. July 21 2016.
Chlorine bromine and iodine are the three common Group 7 elements. If we put a small piece of sodium metal in water sodium reacts exothermically with water producing hydrogen and metal hydroxide. They can be used to find out the relative reactivities of metals.
Could gold be like a halogen. When a more reactive metal is put in salt solution of a less reactive metalthen more reactive metal displace less reactive metal from its salt solution. Na H 2 NaH at high temperatures NaH H 2 O NaOH H 2.
When more reactive metal react with solution of less reactive metal salt more reactive metal displace less reactive metal from its salt. Reaction with limited OH-and limited NH 3 Aqueous complex ions react with limited amounts of sodium hydroxide and ammonia to form coloured precipitates. The colours of the precipitates formed can be used to identify the metal ion CuH2O4OH2 s blue ppt CoH2O4OH2 s blue green ppt.
Soobee72pl and 55 more users found this answer helpful. How do Metals react with Solutions of other salt. While exploring what happens when metals come in contact with acids it is apparent that most but not all have some sort of reaction usually forming hydrogen gas.
Hydrogen gas is not evolved when a metal reacts with dilute nitric acid because nitric acid is a strong oxidizing agent. Hydrogen gas forms as the metals react with the acid to form salts. 2Li 2C Li 2 C 2.
The family that combines with metals to form salts is the halogen family. Some metals react with acid and replace hydrogen from the acid. In a displacement reaction a more reactive metal can displace a less reactive metal from its salt solution.
Therefore the reactivity series of metals can be used to predict the reactions between metals and water. Lots of things form salts with alkali metals but perhaps the best known cases are the halogens meaning salt formers -- fluorine chlorine bromine iodine. The result is the production of salts.
Hydrochloric Acid Some metals dissolve in this acid and this results in the formation of hydrogen gas and oxidized metal chlorides. The reaction is often known as a metal displacement reaction.

Groups In The Periodic Table Metal Non Metal Or Metalloid Colour Or Write In The Metals Non Metals And Metalloids Be Sure To Classify Every Element Ppt Download

Iiiiii 1 The Periodic Table Topic 5 Click For Song Bellwork Using Your Rb Pgs 78 83 Define The Following Words In Your Notebook Family Group Periodic Ppt Download

Chapter 2 C Atoms Molecules And Ions 2 2 歐亞書局 2 8 An Introduction To The Periodic Table A Simple Version Of The Periodic Table Is Shown In Fig Ppt Download

Metals Reacting With Water Acids 2 4 1 Edexcel Igcse Chemistry Revision Notes 2019 Save My Exams

Properties Of Metals Metals Are Good Conductors Of

3 Determine The Number Of Protons Neutrons And Electrons And The Mass Of An Element Using The Periodic Table Locating Metals Nonmetals Metalloids Ppt Download

Neutralisation Of Acids And Salt Production 4 2 3 Aqa Gcse Chemistry Revision Notes 2018 Save My Exams

Flame Test Conclusion Ppt Download

The Periodic Table The Periodic Table Organizes The Elements According To How They Combine With Other Elements Chemical Properties The Periodic Table Ppt Download

Periodic Table Grouping Elements Ppt Video Online Download
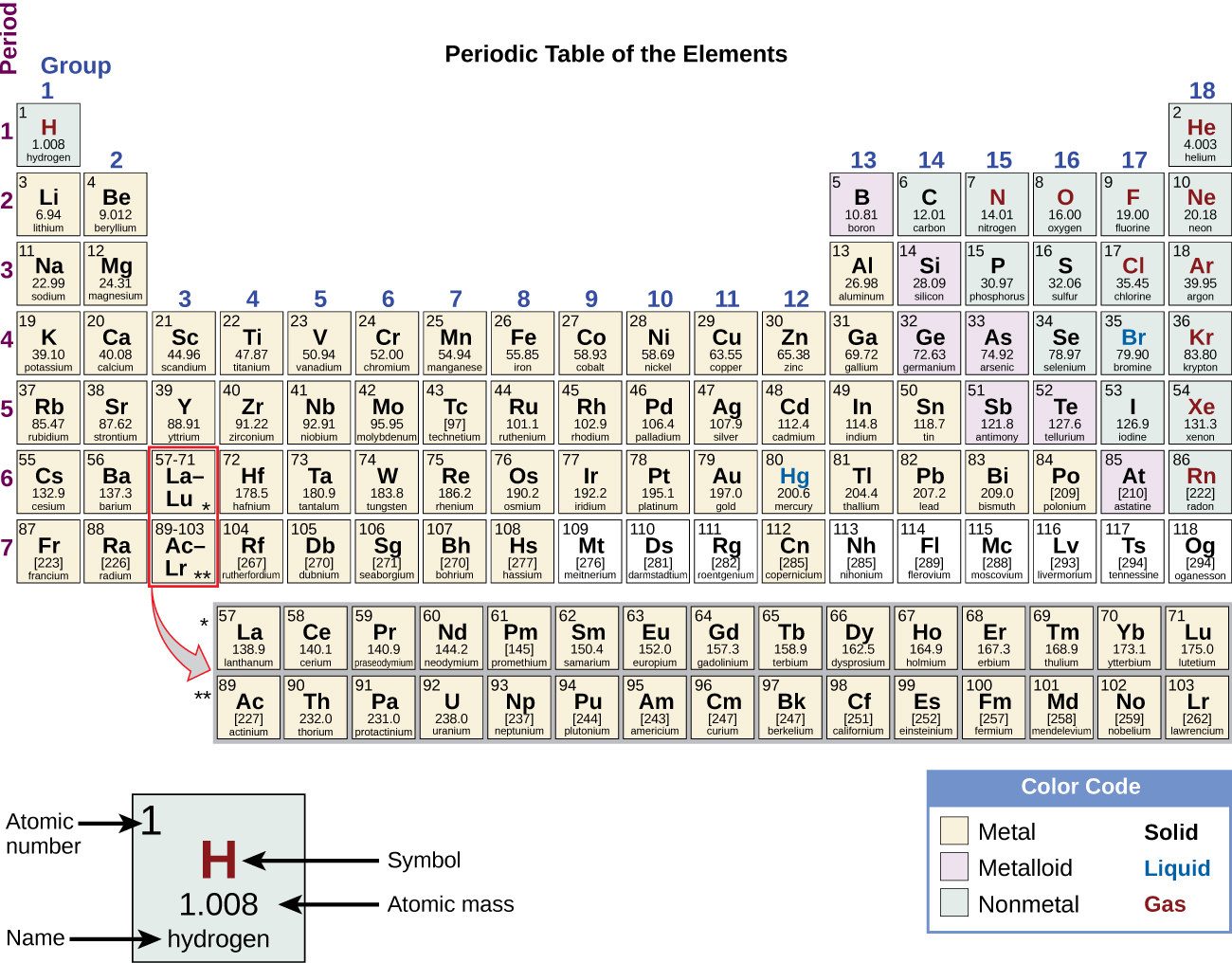
Occurrence Preparation And Properties Of Transition Metals And Their Compounds Chemistry 2e

Flame Test Conclusion Ppt Download
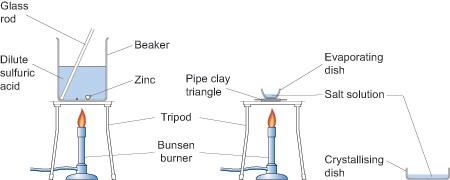
Olcreate Tessa Lbr Module 2 Secondary Science Chemistry Resource 4 Reacting Acids And Metals

Alkali Metal Students Britannica Kids Homework Help

Metal Reactions Dilute Acids Water Oxygen Video Lesson Transcript Study Com

The Halogens Group Vii Known As Halogens Derived From Greek Salt Maker React With Metals To Form Salts Astatine Doesn T Really Exist For A Long Ppt Download

The Organization Of The Periodic Table Answer Key Directions

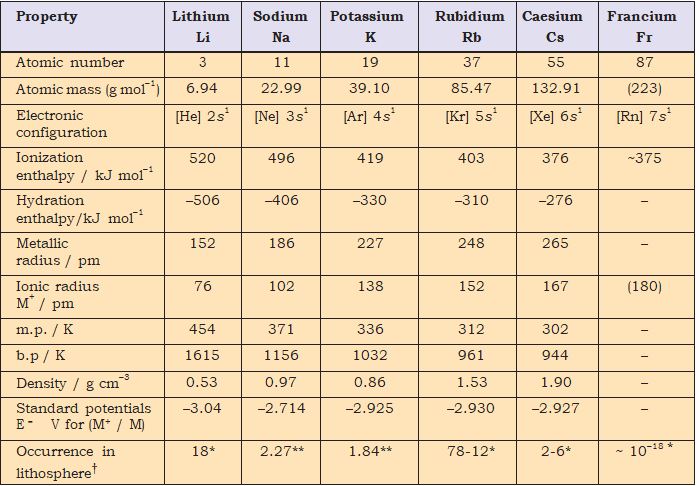
Comments
Post a Comment