Which of the Following Has No Net Dipole Moment
The dipole moment of water is 185 Debye. We offer free revision until our client is satisfied with the work delivered.
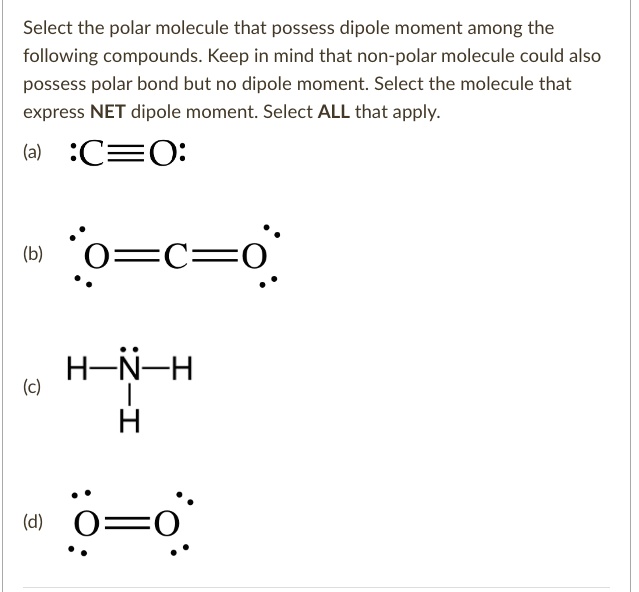
Solved Select The Polar Molecule That Possess Dipole Moment Among The Following Compounds Keep In Mind That Non Polar Molecule Could Also Possess Polar Bond But No Dipole Moment Select The Molecule That Express
The net effect of these microscopic bound currents is to make the magnet behave as if there is a macroscopic electric.
. Normally the dipole moments of the water molecules will be directed randomly and the average dipole moment is zero. The bond dipole is modeled as δ δ with a distance d between the partial charges δ and δ It is a vector parallel to the bond axis. A dipole moment is determined by the magnitudes of the partial charges and by the distances between them.
Dipole moments are reported is units of debye d. 4We calculated the volume averaged dipole moment to derive the material polarization. 5We calculated the material susceptibility.
An HTML5-only web application utilizing jQuery a Java applet a stand-alone Java program Jmoljar and a headless server-side component JmolDatajar. The moments of the two CO bonds cancel each other because of the rectilinear shape of the molecule resulting in a zero net dipole moment in the absence of electric field. Earths magnetic field and magnetic elements.
Bonds add vectorially to give a nonvanishing net dipole moment. Electromagnetic Induction And Alternating Currents. The free revision is offered within 7 days after the assignment has been delivered.
The dipole moment for HCl is small. The bond dipole moment uses the idea of electric dipole moment to measure the polarity of a chemical bond within a moleculeIt occurs whenever there is a separation of positive and negative charges. Both the torque and force exerted on a magnet by an external magnetic field are proportional.
The dipole moment of an electron and a proton separated by 1 Å equals. 6We calculated the dielectric function. We do not at any time disclose clients personal information or credentials to third.
1 the magnitude of the separation of charge and 2 the distance between the negative and positive poles of the molecule. Molecules of larger size can be easily polarised. Bar magnet as an equivalent solenoid magnetic field lines.
Therefore the two dipoles cancel each other out to. The difference between polar and nonpolar molecules can thus be found by the vectors of partial charge resulting from each bond. The polarity of a molecule can be determined by measuring a quantity known as the dipole moment which depends on two factors.
For example hexane will not heat whereas acetone with a dipole moment of. Figure 42 shows a dipole. High polarisability increases the strength of attractive interactions.
4 having a permanent electric dipole moment. But q2l is defined as the dipole moment of the Net force multiple choice questions MULTIPLE CHOICE QUESTIONS-I 1. An interactive viewer for three-dimensional chemical structures.
With no electric field the individual dipoles point in random directions so the net moment per unit volume is zero. For very accurate work this effect. These multiple choice questions are also helpful for the preparation of other competitive exams like UPSEE 2020 WBJEE etc.
Some molecules are nondipolar possessing no permanent moments. Where μ is the dipole moment of the bond given by μQ x r where Q is the charge and r is the distance of separation. Dipole moment of the solv ent the faster the solvent will heat under microwav e irra- diation.
First there is an extra dipole moment induced because of the forces on the electrons. -Current loop as a magnetic dipole and its magnetic dipole moment. 48 x 10-10 esu 10-8 cm 48 x 10-18 esu cm 48 Debye.
A polar molecule is a molecule in which one end of the molecule is slightly positive while the other end is slightly negative. Therefore these molecules have permanent electric dipole moment. When the water is exposed to an external electric field a torque is exerted on the water molecule and it will try to align its dipole moment with the external electric field.
Choose the one most nearly correct and complete answer and If there is. -Magnetic susceptibility and permeability Hysteresis Electromagnets and permanent magnets. But when an electric field is applied two things happen.
You are guaranteed of confidentiality and authenticity. A force is any push or pull. This is schematically illustrated in Figure 42.
Over 10000000 page views. Mu cos0 -mu cos0 0. Induced dipole moment depends upon the dipole moment present in the permanent dipole and the polarisability of the electrically neutral molecule.
The bond dipole μ is given by. If we imagined the Carbon Dioxide molecule centered at 0 in the XY coordinate plane the molecules overall dipole would be given by the following equation. Thus CH 4 is a nonpolar molecule.
The term magnetic moment normally refers to a systems magnetic dipole moment which produces the first term in the multipole expansion of a general magnetic field. A common example is the CO2 molecule in Fig41b. By using our website you can be sure to have your personal information secured.
All the bonds are covalent and there will not be a big dipole moment. This leads to an expression for the susceptibility. Summary of Derivation 2 2 ee e2 0 rr mm mrqE tt 22 e 0 q E r.
This part gives just the same kind of electronic polarizability we found for a nonpolar molecule. To quantitate dipole moments charges are expressed in esus and distances in centimeters. Para- dia- and ferro- magnetic substances.
JmolJSmol is a molecular viewer for 3D chemical structures that runs in four independent modes. 3 acquire a dipole moment only when magnetic field is absent. 3We calculated the electric dipole moment of the charge displaced by r.

Which One Of The Following Compounds Has Non Zero Dipole Moment Youtube

Dipole Moment In This Moment Chemistry Organic Chemistry

Which Of The Following Molecules Does Not Have A Net Dipole Moment Bf3 Brfs Nh3 H2o Homeworklib

Solved 1 Which Of The Following Has No Net Dipole Moment O Chegg Com
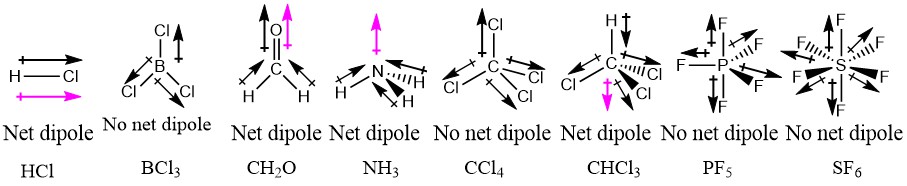
1 4 Polar Covalent Bonds Dipole Moments Chemistry Libretexts

Molecular Dipole The Overall Polarity Of The Molecule Chemistry Steps

Solved Which One Of The Following Molecules Does Not Have A Chegg Com

Question Video Identifying A Polar Molecule From A List Of Molecules Nagwa

Molecular Dipole The Overall Polarity Of The Molecule Chemistry Steps
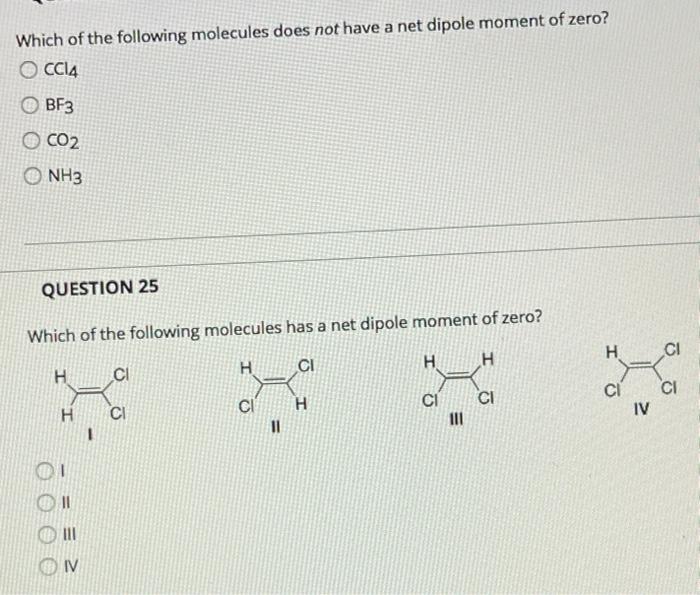
Solved Which Of The Following Molecules Does Not Have A Net Chegg Com

Which Of The Following Alkanes Has No Net Dipole Moment Youtube
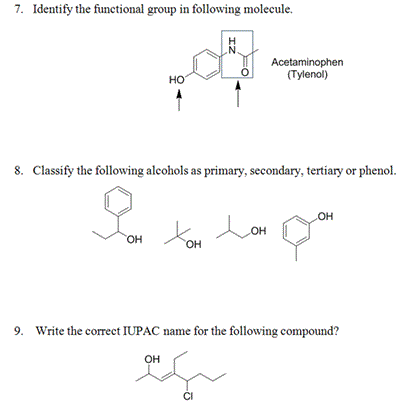
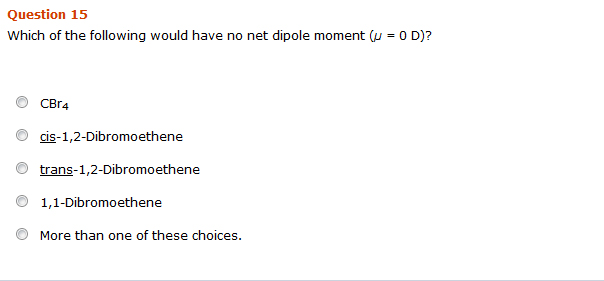
Solved 10 Which Of The Following Would Have No Net Dipole Chegg Com

Dipole Moment Molecular Polarity Percent Ionic Character Youtube
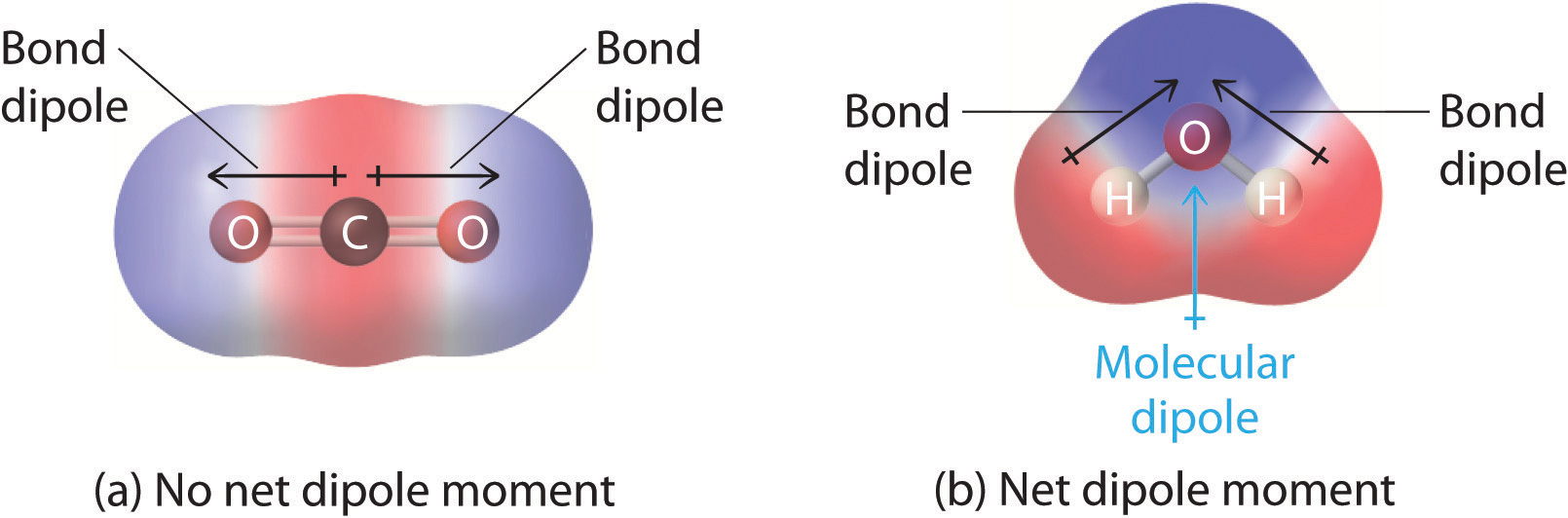
2 2 Polar Covalent Bonds Dipole Moments Chemistry Libretexts
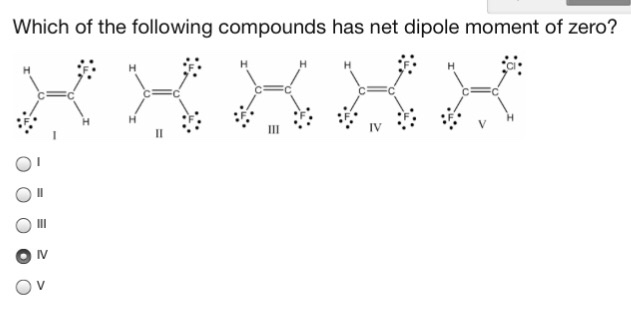
Solved Which Of The Following Compounds Has Net Dipole Chegg Com

Which Of The Following Molecules Does Not Have Net Dipole Moment Youtube

Dipole Moments Mcc Organic Chemistry
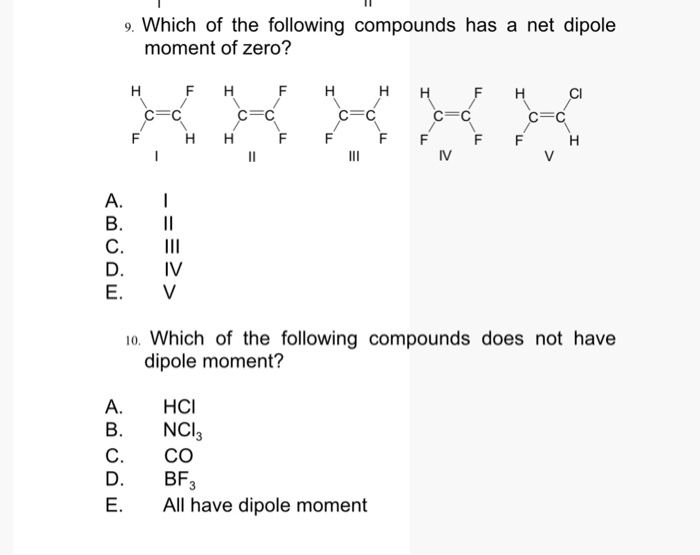
Solved 9 Which Of The Following Compounds Has A Net Dipole Chegg Com

Solved Which Of The Following Would Have No Net Dipole Chegg Com
Comments
Post a Comment